Bank of America just raised its EUR/USD forecast
Investing.com -- Hims & Hers Health (NYSE:HIMS) stock plunged 20% after pharmaceutical giant Novo Nordisk (NYSE:NVO) announced it would terminate its collaboration with the telehealth company over the sale of weight loss drugs.
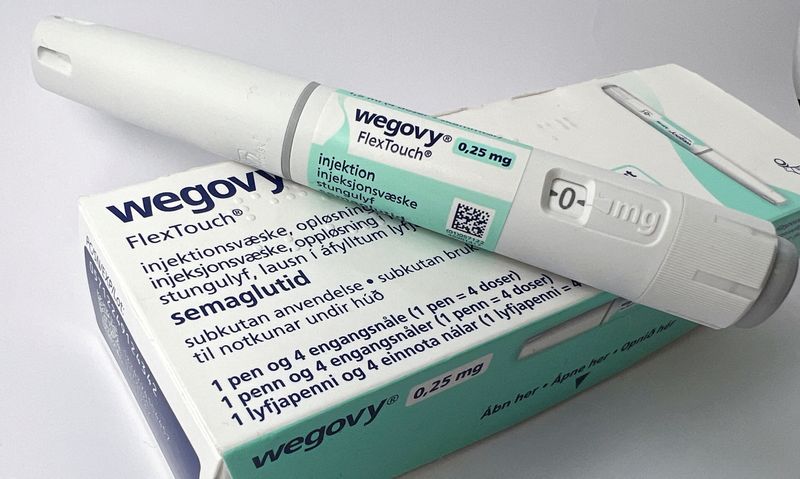
The Danish drugmaker said Monday it would no longer provide Hims & Hers with direct access to its FDA-approved weight loss drug Wegovy through NovoCare Pharmacy. The partnership, which was expanded just last month in April, had aimed to make obesity treatments more accessible and affordable for Americans.
According to Novo Nordisk, the decision comes after Hims & Hers allegedly failed to comply with laws prohibiting mass sales of compounded drugs. The pharmaceutical company accused the telehealth provider of "disseminating deceptive marketing that put patient safety at risk."
"Novo Nordisk is firm on our position and protecting patients living with obesity," said Dave Moore, Executive Vice President of US Operations at Novo Nordisk. "When patients are prescribed semaglutide treatments by their licensed healthcare professional or a telehealth provider, they are entitled to receive authentic, FDA-approved and regulated Wegovy."
Hims & Hers CEO Andrew Dudum responded in a statement on X, calling Novo’s claims "misleading" and stating that Novo’s commercial team was pressuring the company to "steer patients to Wegovy." "We refuse to be strong-armed by any pharmaceutical company’s anticompetitive demands," he said, affirming that Wegovy will still be available on the Hims & Hers platform.
The original collaboration, announced in April, had offered Americans access to Wegovy through the Hims & Hers platform at a unified price starting at $599 per month, which included the medication along with 24/7 care, clinical support, and nutrition guidance.
Novo Nordisk expressed concerns about "knock-off drugs" made with foreign ingredients, specifically citing suppliers from China that have not been authorized by the FDA to manufacture semaglutide, the active ingredient in Wegovy.