AlphaTON stock soars 200% after pioneering digital asset oncology initiative
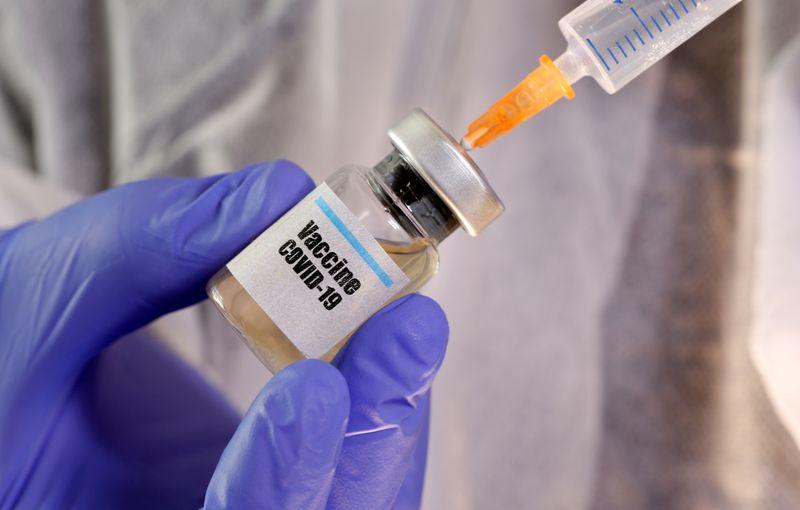
CAMBRIDGE, MA - Moderna, Inc. (NASDAQ:MRNA), currently valued at $10.2 billion, has announced its submission of an updated COVID-19 vaccine formula, Spikevax 2025-2026, for review by the U.S. Food and Drug Administration (FDA). The new vaccine targets the SARS-CoV-2 variant LP.8.1 and aligns with the FDA’s guidance for COVID-19 vaccines to be updated to the monovalent JN.1 lineage, with a preference for the LP.8.1 variant. According to InvestingPro data, the company maintains a strong liquidity position with a current ratio of 4.22, though it faces challenges with revenue projected to decline this year.
The submission reflects the ongoing efforts to adapt to the evolving landscape of the pandemic and to provide vaccines that are effective against emerging variants of the virus. Moderna’s announcement comes as part of its commitment to leveraging its mRNA platform to respond rapidly to new strains of the virus. The company’s stock has experienced significant volatility, with shares down over 80% in the past year, though InvestingPro analysis suggests the stock may be undervalued at current levels.
As per the authorized use in the United States, the SPIKEVAX vaccine is indicated for active immunization to prevent COVID-19 in individuals 12 years of age and older. Moderna’s vaccine has been a critical tool in the global fight against the pandemic, with a wide range of applications in various populations.
The company has outlined safety information, including contraindications for individuals with a history of severe allergic reactions to any component of the vaccine. It has also noted the management of acute allergic reactions and the increased risks of myocarditis and pericarditis, which have been observed particularly in younger male adults.
The most commonly reported adverse reactions include pain at the injection site, headache, fatigue, myalgia, and fever, among others. Healthcare providers are responsible for reporting any adverse events to the Vaccine Adverse Event Reporting System (VAERS).
While the press release contains forward-looking statements regarding the potential approval of the updated Spikevax formula in the U.S., these statements are not guarantees of future performance and are subject to risks and uncertainties. InvestingPro reveals that 6 analysts have revised their earnings upwards for the upcoming period, though the company is currently burning through cash with negative free cash flow of $4 billion in the last twelve months. For deeper insights into Moderna’s financial health and future prospects, investors can access comprehensive analysis through InvestingPro’s detailed research reports, available for over 1,400 US stocks.
This news is based on a press release statement from Moderna, Inc., and provides a summary of the key facts regarding the company’s submission to the FDA. The review process by the FDA will determine the availability of the updated vaccine for the upcoming flu season.
In other recent news, Moderna has announced the withdrawal of its application for the flu/COVID combination vaccine, mRNA-1083, in consultation with the FDA. The company plans to resubmit the application later this year, incorporating new data from an ongoing Phase 3 trial of its seasonal influenza vaccine, mRNA-1010. Meanwhile, Brookline Capital Markets has maintained its Buy rating for Moderna, with a price target of $198, emphasizing confidence in the company’s timeline and the potential market success of mRNA-1083 by 2026. In a separate development, Berenberg has reaffirmed its Hold rating on Moderna with a $30 price target, following the company’s mixed earnings report that highlighted effective cost management amid commercial challenges. The firm noted Moderna’s efforts to cut costs but suggested that more aggressive actions might be needed to meet long-term financial goals. Additionally, the FDA is requiring new clinical trials for COVID-19 boosters for those under 65, which may impact vaccine availability for younger, healthier populations. Moderna, alongside other vaccine manufacturers, may face delays in booster approvals due to this requirement. Lastly, the Department of Health and Human Services is expected to announce changes to CDC’s recommendations for routine COVID-19 vaccines for children and pregnant women, potentially affecting vaccine producers like Moderna.
This article was generated with the support of AI and reviewed by an editor. For more information see our T&C.